| |
Product(s): |
WaterGEMS, WaterCAD |
|
| |
Version(s): |
CONNECTION Edition, V8i 08.11.05.61 and greater |
|
| |
Area: |
Modeling |
|
|
Author: |
Dr. Tom Walski |
|
Background
The problem with mixing chlorine (free chlorine, HOCl) with chloramines (combined chlorine, usually monochloramine, NH2Cl ) is that they combine to form species with little disinfectant value (nitrogen trichlorinde, NCl3).
This can be written as
2 HOCl + NH2Cl à NCl3 + 2 H2O
If you have a mixture with a little free chlorine and a lot of chloramine, the chloramine can still be an effective disinfectant. If you have a mixture with a lot of free chlorine and a little chloramine, the free chlorine can still be an effective disinfectant. However, in a system where free chlorine and chloramines mix, there will be a front where the two disinfectants essentially cancel each other out. Without interferences, it would take 2 moles of chlorine to react with 1 mole of ammonia. The worst condition is called breakpoint and there is very little disinfectant at that point as shown in the figure below. This front can move around during the day depending on operation.
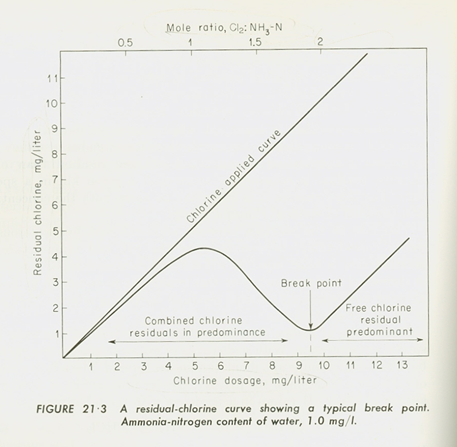
For example, you could mix water with 2 mg/L of free chlorine with water with 2 mg/L chloramines and end up with water with essentially none of either—not a desirable situation. The chemical balance above illustrated that case. In most cases, there will be an excess of free chlorine or chloramnes.
Multi-Species Extension (MSX)
With source tracing in WaterGEMS and WaterCAD, we can determine how far each water source moves into the system. To get a clear picture of the different species, we would need to use the Multi-Species Extension (MSX) available starting with version 08.11.05.61. You would need to define the equations for chlorine and chloramine decay and reaction between them to form nitrogen trichloride. The problem is complicated by the fact that there are other interfering species such that a straight stoichiometric balance may not be quite right.
The [TERMS] section of the MSX setup can be used to define the equations and parameters and reference hydraulic variables like the Reynolds number. See more here: Adding custom parameters and equations with Multi Species Extension (MSX) Also see related forum discussion here.
It would help to do some bottle tests in the lab to verify the equations. The reactions may be fast enough that reaction rates may not be a concern. You would then make runs with MSX and see if the model correctly predicts the species that result.
Determining Diffusivity of Chloramines
Determining the diffusivity of Chloramines may be difficult. Historically, one source of values for things like this is the Perry's handbook of Chemistry or CRC Tables.
Depending on the relative size of diffusivity vs, advection, often the diffusivity is negligible. So, a sensitivity analysis may help; start with chlorine and see how sensitive results are to this parameter.
One thing to consider is that a regular constituent analysis may not work well for Chloramine. As mentioned further above, you may want to look into the Multi-species Extension (MSX). You can read more about MSX in the Help and here.
See Also
What's New with WaterGEMS/WaterCAD V8i SELECTSeries 5
Dm for monochloramine?